Abstract
Background: Lovo-cel (bb1111; LentiGlobin for sickle cell disease [SCD]) gene therapy (GT) uses autologous transplantation of hematopoietic stem and progenitor cells (HSPCs) transduced with the BB305 lentiviral vector encoding a modified β-globin gene, which produces an anti-sickling hemoglobin (Hb), HbAT87Q. The ongoing phase 1/2 HGB-206 study (NCT02140554) is the largest clinical trial of GT in SCD to date. We report updated efficacy and safety results for Group C and describe the results of additional investigations in two patients (pts) in Group C with persistent anemia.
Methods: Pts (≥12-≤50 yrs of age) with SCD and recurrent severe vaso-occlusive episodes (VOEs) underwent plerixafor mobilization and apheresis followed by myeloablative busulfan conditioning and lovo-cel infusion. Lab evaluations, SCD-related outcomes, and safety were assessed. Data are median (min-max) unless otherwise stated; all timepoints are from lovo-cel infusion.
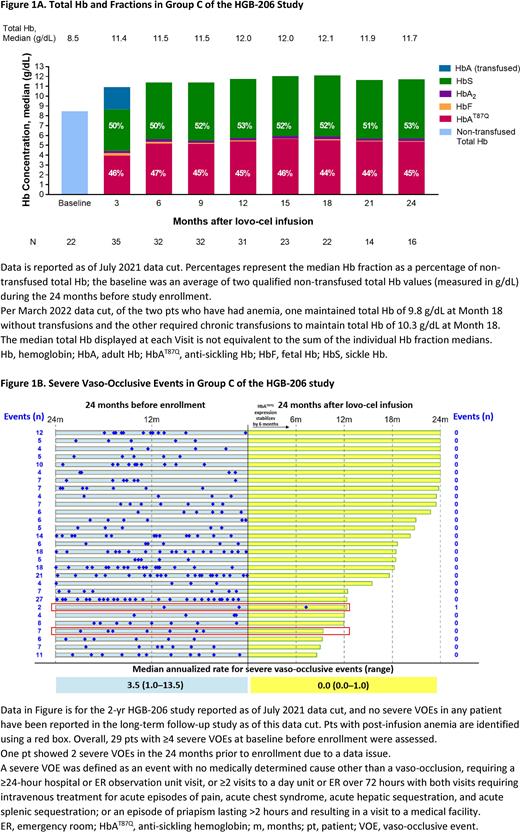
Results: As of July 2021, 35 Group C pts had received a lovo-cel infusion, with a median follow-up of 20.9 months (min-max: 8.5-28.5). Neutrophil and platelet engraftment were achieved on Days 20 (12-35) and 36 (18-136), respectively. The median vector copy number in peripheral blood remained stable in all pts at ≥1 c/dg from 6 months after infusion until the last study visit, and resulted in median HbAT87Q levels of ≥5 g/dL during this period. The median total Hb level increased without transfusions from 8.5 g/dL at baseline to ≥11 g/dL from 6 months through last visit; HbAT87Q contributed at least 40% of total Hb (Fig 1A). Of the 29 pts who could be evaluated, 28 had a complete resolution of severe VOEs after lovo-cel infusion in the 2-yr parent study as of last follow-up, as compared with 3.5 events per yr (1.0-13.5) in the 2 yrs before enrollment (Fig. 1B); no severe VOEs have been reported during the long-term follow up study. Key hemolysis markers approached normal levels.
Overall, 15 pts (43%) had ≥1 serious adverse event after lovo-cel infusion; the most frequently reported were abdominal pain, drug withdrawal syndrome (opiate), nausea, vomiting, and dehydration (6% each). No cases of veno-occlusive liver disease, graft failure, replication-competent lentivirus, vector-mediated insertional oncogenesis, or hematologic malignancies were observed. The lovo-cel treatment regimen generally reflected the known side effects of HSPC collection and busulfan conditioning regimen, and/or adverse events commonly seen in the population being evaluated.
Further investigations were conducted as of March 2022 in two pts who presented with similar clinical and pathological findings that initially raised suspicion of myelodysplastic syndrome (MDS). Presentation features included neutropenia in one patient; both patients had persistent anemia despite adequate HbAT87Q production, bone marrow morphology showing erythroid specific dysplasia (binucleated or trinucleated cells) consistent with dyserythropoiesis as seen in other hemoglobinopathies, and low level of trisomy 8 (≤7.7%) only observed by fluorescence in situ hybridization analysis. There are no clinical symptoms suggestive of hematologic malignancies, no driver mutations, no clonal process (vector-related or otherwise) as shown by Integration Site Analysis and Next-Generation Sequencing (both subjects had >29,000 Unique Integration Sites with no integration having a relative frequency above 0.425%), and no chromosomal abnormalities on karyotyping among up to 205 metaphases. Both pts have had anemia and one maintained total Hb of 9.8 g/dL at Month 18 without transfusions, and the other required chronic transfusions to maintain 10.3 g/dL at Month 18 with otherwise normal complete blood counts. Both pts experienced a reduction or resolution of severe VOEs after lovo-cel infusion. Of note, these are the only pts in the study to have two α-globin deletions (−α3.7/−α3.7). We propose that α-globin trait may contribute to anemia and dyserythropoiesis after lovo-cel infusion.
Summary: In Group C of the HGB-206 study, one-time treatment with lovo-cel resulted in sustained production of HbAT87Qand near-complete resolution of severe VOEs. Investigations to date in both patients with anemia have not confirmed MDS; thus, currently no hematologic malignancies have occurred in Group C. Ongoing long-term follow-up should be continued for all pts receiving GT.
Disclosures
Walters:Ensoma, Inc.: Consultancy, Current holder of stock options in a privately-held company; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; BioLabs, Inc.: Consultancy; AllCells, Inc.: Consultancy. Thompson:bluebird bio, Inc.: Consultancy, Research Funding; Beam: Consultancy; Agios: Consultancy; Novartis: Research Funding; Baxalta: Research Funding; Biomarin: Research Funding; Editas: Research Funding; Global Blood Therapeutic: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Research Funding; CRISPR/Vertex: Consultancy, Research Funding. Kwiatkowski:Agios: Consultancy; bluebird bio, Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Imara: Consultancy, Research Funding; Forma: Consultancy, Research Funding; Chiesi: Consultancy; Silence Therapeutics: Consultancy; Apopharma: Research Funding; Bioerativ: Research Funding; Sangamo: Research Funding; Biomarin: Consultancy; CRISPR/Vertex: Research Funding. Mapara:Ossium: Consultancy. Rifkin-Zenenberg:Global Blood Therapeutics: Honoraria; Aruvant: Honoraria. Aygun:National Heart, Lung, Blood Institute: Research Funding; National Institute of Nursing Research: Research Funding; Patient Centered Outcomes Research Institute: Research Funding; bluebird bio, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Kasow:Aruvant: Consultancy, Membership on an entity's Board of Directors or advisory committees. Miller:bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Zhang:Solution-Stat Inc.: Other: Business owner; Cytel: Other: Contractor; bluebird bio, Inc.: Current Employment; GlobalTeq Consulting Inc.: Other: Contractor. Chawla:bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Macari:CAMP4 Therapeutics: Current holder of stock options in a privately-held company; bluebird bio, Inc.: Current Employment. Pierciey:bluebird bio, Inc.: Current Employment. Kanter:Bausch: Consultancy, Honoraria; GLG: Consultancy; Fulcrum Tx: Consultancy; Forma: Consultancy, Membership on an entity's Board of Directors or advisory committees; University of Alabama Birmingham: Current Employment; Novartis: Consultancy, Honoraria; Ecor1: Honoraria; Guidepoint Global: Honoraria; Beam: Honoraria; Graphite Bio: Consultancy; ORIC: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal